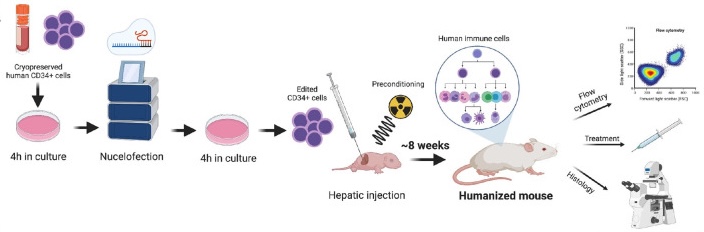
Human CD34+ hematopoietic stem and progenitor cells (HSPCs) are a standard source of cells for clinical HSC transplantations as well as experimental xenotransplantation to generate “humanized mice”. To further extend the range of applications of these humanized mice, we developed a protocol to efficiently edit the genomes of human CD34+ HSPCs before transplantation. In the past, manipulating HSPCs has been complicated by the fact that they are inherently difficult to transduce with lentivectors, and rapidly lose their stemness and engraftment potential during in vitro culture. However, with optimized nucleofection of sgRNA:Cas9 ribonucleoprotein complexes, we are now able to edit a candidate gene in CD34+ HSPCs with almost 100% efficiency, and transplant these modified cells in immunodeficient mice with high engraftment levels and multilineage hematopoietic differentiation. The result is a humanized mouse from which we knocked out a gene of interest from their human immune system.


