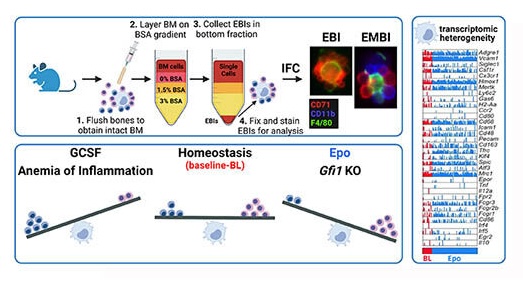

The erythroblastic island (EBI), composed of a central macrophage surrounded by maturing erythroblasts, is the erythroid precursor niche. Despite numerous studies, its precise composition is still unclear. Using multispectral imaging flow cytometry (IFC), in vitro island reconstitution, and single cell RNA-sequencing (scRNA-seq) of adult mouse bone marrow EBI-component cells enriched by gradient sedimentation, we present evidence that the CD11b+-cells present in the EBIs are neutrophil precursors specifically associated with bone marrow EBI macrophages, indicating that erythro-(myelo)-blastic islands are a site for both terminal granulopoiesis and erythropoiesis. We further demonstrate that the balance between these dominant and terminal differentiation programs is dynamically regulated within this bone marrow niche by pathophysiological states, favoring granulopoiesis during anemia of inflammation, or erythropoiesis after erythropoietin (Epo) stimulation. Finally, by molecular profiling, we reveal the heterogeneity of EBI macrophages by Cellular Indexing of Transcriptome and Epitopes (CITE)-sequencing of mouse bone marrow EBIs at baseline and after Epo-stimulation in vivo and provide a searchable, online viewer of this data characterizing the macrophage subsets serving as hematopoietic niches. Taken together, our findings demonstrate that EBIs serve a dual role as niches for terminal erythropoiesis and granulopoiesis and the central macrophages adapt to optimize production of red blood cells or neutrophils.
This work was supported by U54 DK126108 and Flow Cytometry and Single Cell Characterization and Procurement performed at the Cincinnati Center of Excellence in Molecular Hematology Cores at Cincinnati Children’s Hospital Medical Center.
