Precision analysis of mutant U2AF1 activity reveals deployment of stress granules in myeloid malignancies
Abstract Text:
This work was supported by a Pilot and Feasibility type B grant from U24 DK126127


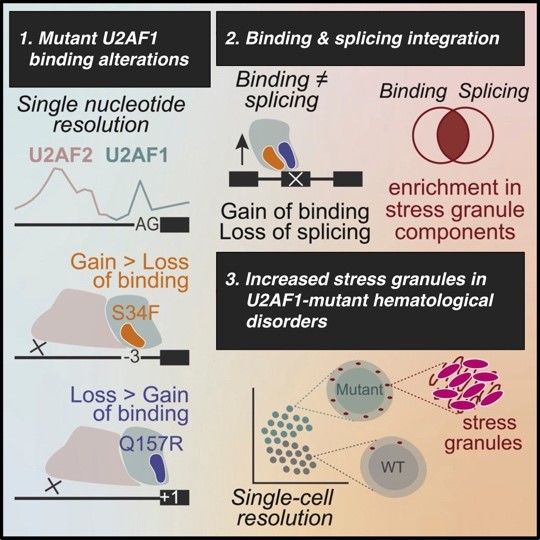
Splicing factor mutations are common among cancers, recently emerging as drivers of myeloid malignancies. U2AF1 carries hotspot mutations in its RNA-binding motifs; however, how they affect splicing and promote cancer remain unclear. The U2AF1/U2AF2 heterodimer is critical for 3′ splice site (3’SS) definition. To specifically unmask changes in U2AF1 function in vivo, we developed a crosslinking and immunoprecipitation procedure that detects contacts between U2AF1 and the 3’SS AG at single-nucleotide resolution. Our data reveal that the U2AF1 S34F and Q157R mutants establish new 3’SS contacts at -3 and +1 nucleotides, respectively. These effects compromise U2AF2-RNA interactions, resulting predominantly in intron retention and exon exclusion. Integrating RNA binding, splicing, and turnover data, we predicted that U2AF1 mutations directly affect stress granule components, which was corroborated by single-cell RNA-seq. Remarkably, U2AF1-mutant cell lines and patient-derived MDS/AML blasts displayed a heightened stress granule response, pointing to a novel role for biomolecular condensates in adaptive oncogenic strategies.
This work was supported by a Pilot and Feasibility type B grant from U24 DK126127