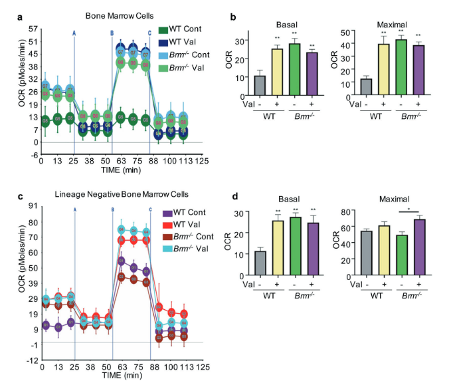
Little is known of hematopoietic stem (HSC) and progenitor (HPC) cell self-renewal. The role of Brahma (BRM), a chromatin remodeler, in HSC function is unknown. Bone marrow (BM) from Brm-/- mice manifested increased numbers of long- and short-term HSCs, GMPs, and increased numbers and cycling of functional HPCs. However, increased Brm-/- BM HSC numbers had decreased secondary and tertiary engraftment, suggesting BRM enhances HSC self-renewal. Valine was elevated in lineage negative Brm-/- BM cells, linking intracellular valine with Brm expression. Valine enhanced HPC colony formation, replating of human cord blood (CB) HPC-derived colonies, mouse BM and human CB HPC survival in vitro, and ex vivo expansion of normal mouse BM HSCs and HPCs. Valine increased oxygen consumption rates of WT cells. BRM through CD98 was linked to regulated import of branched chain amino acids, such as valine, in HPCs. Brm-/- LSK cells exhibited upregulated interferon response/cell cycle gene programs. Effects of BRM depletion are less apparent on isolated HSCs compared to HSCs in the presence of HPCs, suggesting cell extrinsic effects on HSCs. Thus, intracellular valine is regulated by BRM expression in HPCs, and the BRM/valine axis regulates HSC and HPC self-renewal, proliferation, and possibly differentiation fate decisions.
