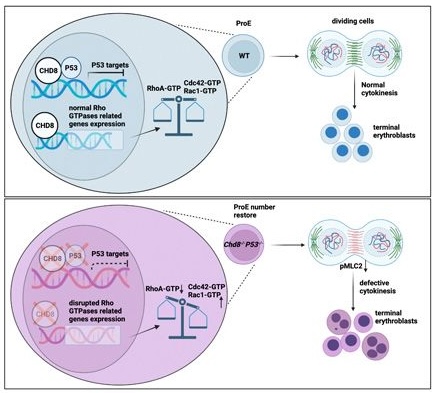
CHD8 is an ATP-dependent chromatin-remodeling factor whose monoallelic mutation defines a subtype of autism spectrum disorders (ASDs). Previous work found that CHD8 is required for the maintenance of hematopoiesis by integrating ATM-P53-mediated survival of hematopoietic stem/progenitor cells (HSPCs). Here, by using Chd8F/FMx1-Cre combined with a Trp53F/F mouse model that suppresses apoptosis of Chd8-/- HSPCs, we identify CHD8 as an essential regulator of erythroid differentiation. Chd8-/-P53-/- mice exhibited severe anemia conforming to congenital dyserythropoietic anemia (CDA) phenotypes. Loss of CHD8 leads to drastically decreased numbers of orthochromatic erythroblasts and increased binucleated and multinucleated basophilic erythroblasts with a cytokinesis failure in erythroblasts. CHD8 binds directly to the gene bodies of multiple Rho GTPase signaling genes in erythroblasts, and loss of CHD8 results in their dysregulated expression, leading to decreased RhoA and increased Rac1 and Cdc42 activities. Our study shows that autism-associated CHD8 is essential for erythroblast cytokinesis.
This work was partially supported by Cincinnati Children’s Hospital Center of Excellence Cores U54 DK126108.

